An Overview of Clinical Trials
Clinical trials aim to test whether different treatments are safe and how well they work. They are often used to test new medicines, but they can also be used to look at new ways of using existing medicines. They are carried out in a number of phases.
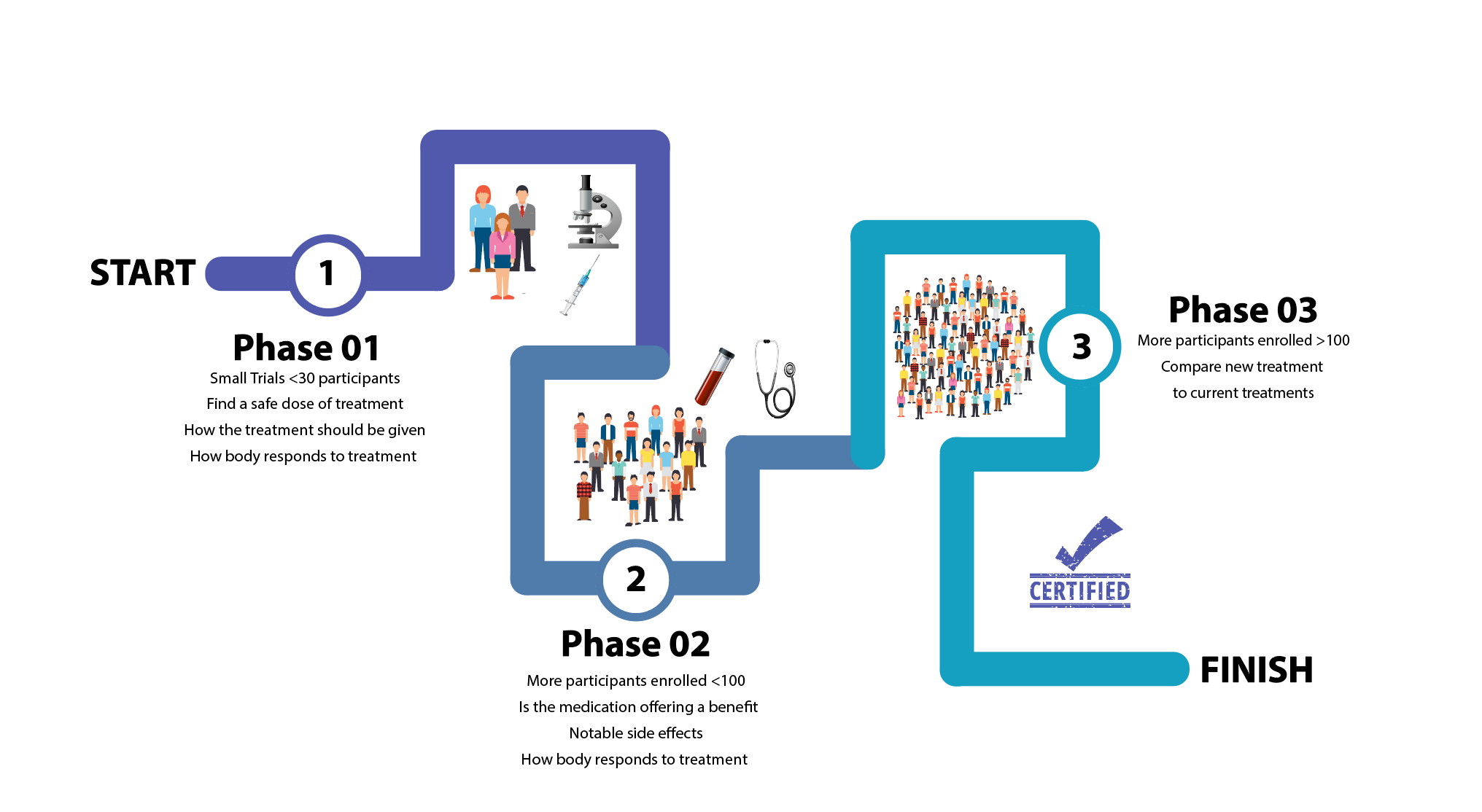
- Phase 1: This is the first stage of a clinical trial and it involves a small group of participants, usually healthy people without the disease. It usually aims to find out how safe a drug is and if it is targeting the right thing in the body.
- Phase 2: When a clinical trial reaches Phase 2, the researchers often know quite a lot about the new drug. The purpose of a Phase 2 clinical trial is to test the drug in a larger number of people, now including people with the disease, to have a better understanding of its safety and what the correct dose of the medication is. In this phase researchers are also looking to see if the drug may be having a benefit to participants.
- Phase 3: When a clinical trial reaches Phase 3, the new drug is looking promising. These trials usually include hundreds of participants usually from several different countries. Phase 3 trials can last a long time as they aim to assess the real benefit of the new drug. They may look to compare the effects of the new drug with standard drugs or treatment practice. In this phase researchers aim to find out more about how common side effects are and whether there are any possible long term problems that may develop through taking the new medication. If a Phase 3 trial is proven to be successful it will be licensed for use. Monitoring of the new drug still happens to learn more about the long-term benefits and risks and any rare side effects that occur.
Current Status of Clinical Trials
| Company | Target | Program | Current stage |
|---|---|---|---|
| Alector | Progranulin | INFRONT-3 AL001 | Phase 3 |
| Prevail | Progranulin | PROCLAIM J4B-MC-OKAA (LY3884963) | Phase 1/2 |
| Passage Bio | Progranulin | upliFT-D PBFT02 | Phase 1/2 |
| Denali | Progranulin | DNL593 | Phase 1/2 |
| Aviado Bio | Progranulin | ASPIRE-FTD AVB-101 | Phase 1/2 |
| Vesper Bio | Progranulin | VES001 | Phase 1 |
| Transposon Therapeutics | C9orf72 | TPN-101 | Phase 2 |
| OrphAI Therapeutics | C9orf72 | AIT-101 (LAM-002A) | Phase 2 |
| Single site academic-led study at University of Florida | C9orf72 | Metformin | Phase 2 |
We are currently helping to run the following trials at UCL:
- Alector (no longer recruiting)
- Prevail (recruitment on hold)
We will expect to be additionally running the following trials soon:
- Aviado Bio
- Vesper Bio
Please contact our team at genfi@ucl.ac.uk if you are interested in taking part in clinical trials.
For people with ALS alone, there have been many more trials that have happened and a number of ongoing studies. The I am ALS website has a Clinical Research Dashboard which keeps an updated list of clinical trials for people with ALS.